technology
technology
Innovation in Every Pulse


How a Lead Acid Battery Works
A lead-acid battery is like a small chemical factory that stores energy in its plates. It’s filled with a mixture of pure sulfuric acid and distilled water, known as the electrolyte. When the lead dioxide (PbO2) plate and the sponge lead (Pb) plate are placed in this electrolyte, a chemical reaction occurs, generating electrical energy.
During the discharge cycle, the active lead from the positive plate reacts with the sulfate in the electrolyte to form lead sulfate (PbSO4) on the positive plate. Meanwhile, on the negative plate, the lead combines with the sulfate to form lead sulfate as well. This process reduces the battery’s charge.
When recharging, the process reverses. The lead sulfate on both plates breaks down back into lead and sulfate. The water in the electrolyte splits into hydrogen and oxygen, and the sulfate (SO4) recombines with hydrogen (H2) to restore the electrolyte.
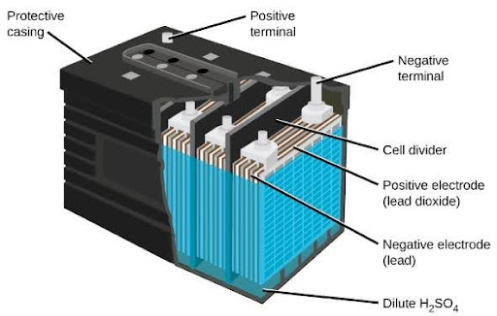
In simple terms, during discharge, the electrolyte’s specific gravity decreases. Once the battery is fully charged, the specific gravity returns to its original value of 1.260-1.270.
How Battery Regeneration Works
A lead-acid battery is like a small chemical factory that stores energy in its plates. It’s filled with a mixture of pure sulfuric acid and distilled water, known as the electrolyte. When the lead dioxide (PbO2) plate and the sponge lead (Pb) plate are placed in this electrolyte, a chemical reaction occurs, generating electrical energy.
This resonant frequency helps the lead plates to safely shed the harmful sulfate deposits. These sulfate build-ups dissolve
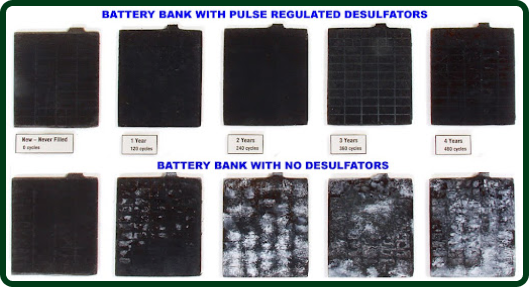
back into the battery’s electrolyte, thereby increasing the battery’s specific gravity and voltage while reducing its impedance to factory levels. As a result, the overall performance of the battery is significantly enhanced.
Revivepoint’s battery regeneration technology effectively tackles hard sulfation by breaking down the crystals and converting them into amorphous forms. Achieving this level of efficiency is made possible through advanced regenerator machines and their accompanying software.
The combination of high efficiency, automation, and long-term support ensures optimal results in battery restoration and regeneration.
Battery reconditioning can be performed regularly as part of a maintenance service or as a one-time full service.
Why Batteries Die
Batteries often die due to a process called sulfation. Sulfation happens when lead sulfate crystals form on the surface and within the pores of the battery’s lead plates. If left untreated, these crystals grow larger and reduce the battery’s capacity until it can no longer function.
Normally, during battery use, lead sulfate crystals form temporarily and dissolve during recharging. However, problems arise when these crystals become permanent. This irreversible sulfation occurs when some sulfate crystals don’t return to the electrolyte during charging and stay attached to the battery plates. This issue worsens if the battery stays undercharged or idle for long periods.
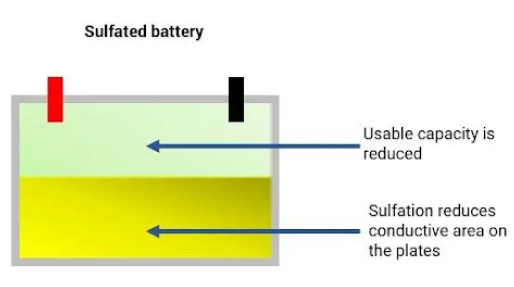
Over time, the battery plates become clogged with these crystals, creating an insulating layer between the electrolyte and the plates. This insulation prevents proper charging, leading to increased internal resistance and decreased electrolyte specific gravity. As a result, fewer sulfate ions are available for chemical reactions, causing the battery to fail prematurely.
In summary, the combination of clogged, sulfated plates and weak electrolyte contributes to the early failure of the battery.
Benefits of Battery Regeneration
-
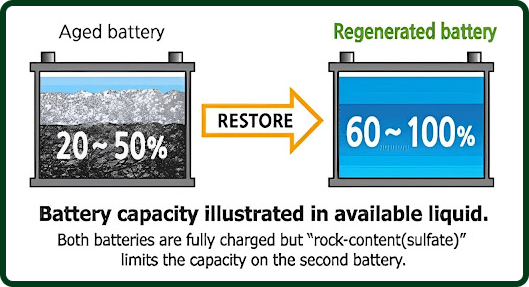
A new battery starts with 100% capacity, which drops to 70% after 2-3 years and 45% after 4-5 years. Regenerated batteries can achieve up to 95% capacity. -
Older batteries take up to 40% longer to charge. Regenerated batteries charge almost as quickly as new ones. -
Battery regeneration helps preserve nature by reducing waste and pollution. -
Each lead-acid battery contains 2-3 liters of toxic sulfuric acid and lead. -
Lead is a cumulative poison harmful to humans, animals, and plants. -
Regenerating batteries reduces the need for new battery investments.
-
Regenerated batteries perform like new, providing full power. -
Battery regeneration extends the life of batteries. -
Saves on the cost of replacing old batteries. -
Reduces the need for new raw materials. -
Decreases plastic and chemical waste. -
Helps reduce the impact on climate change.
